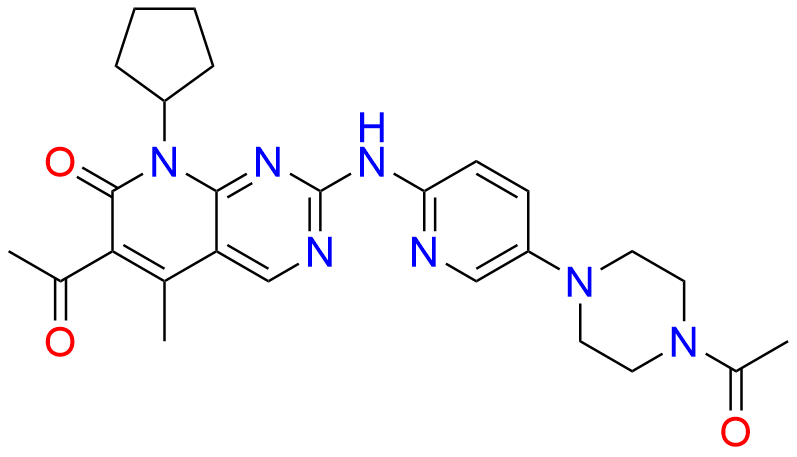
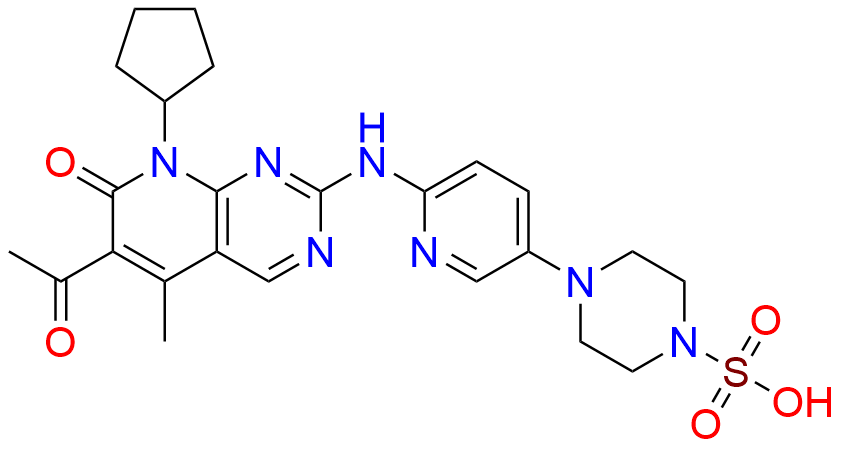
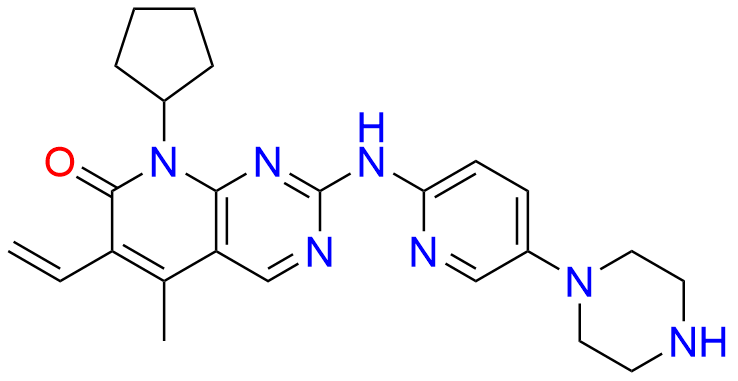
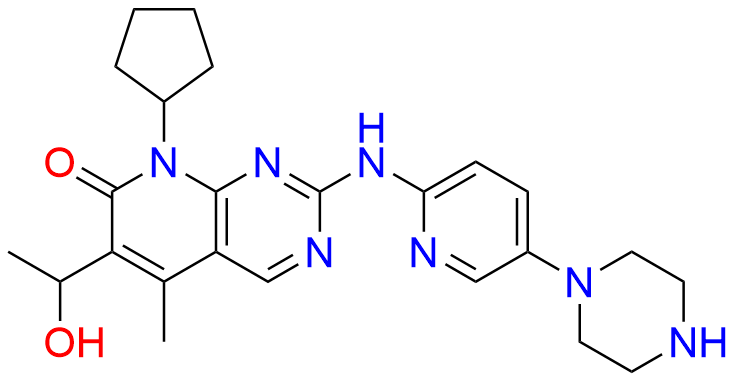
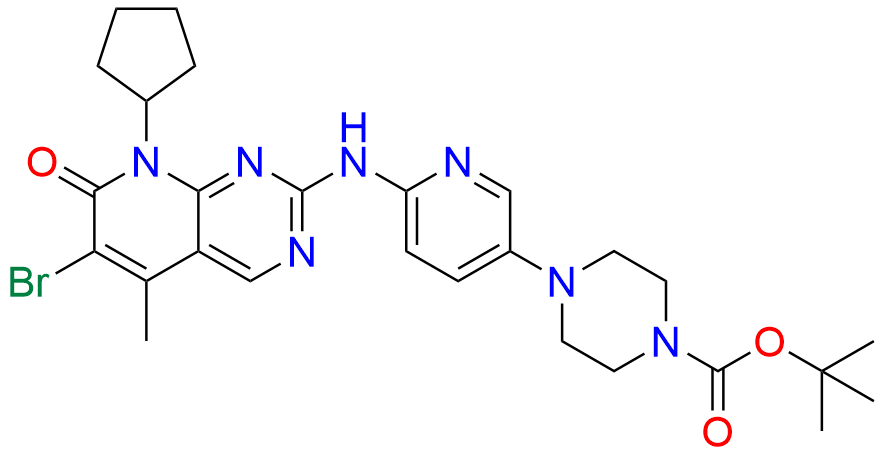
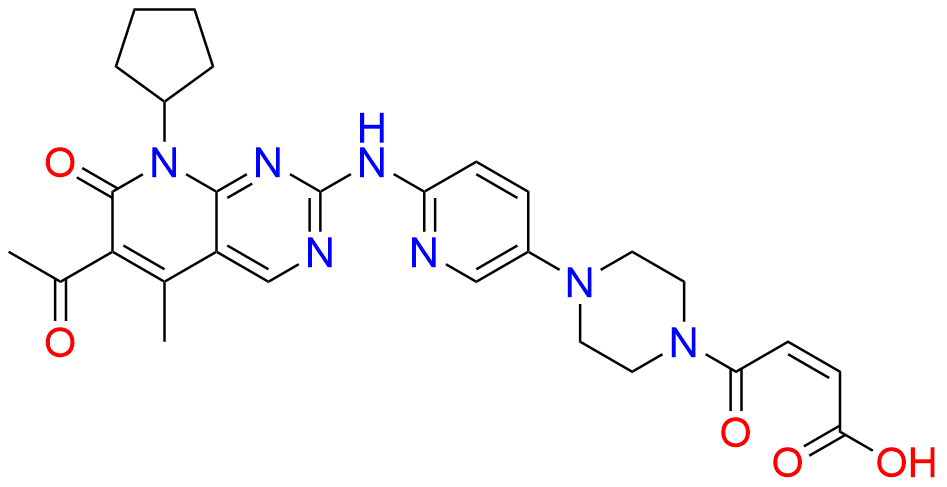
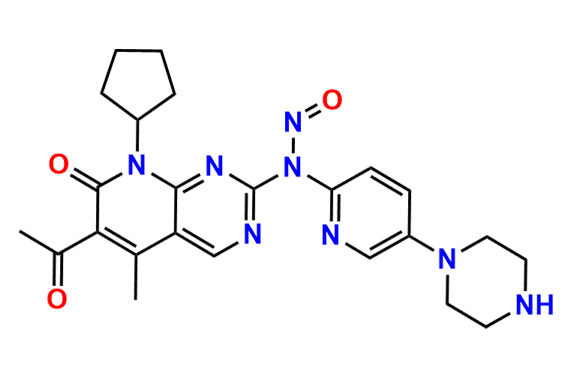
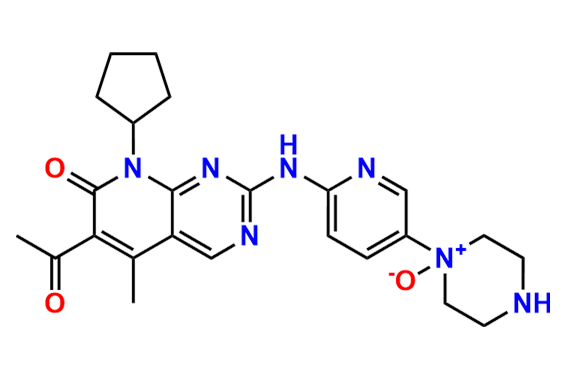
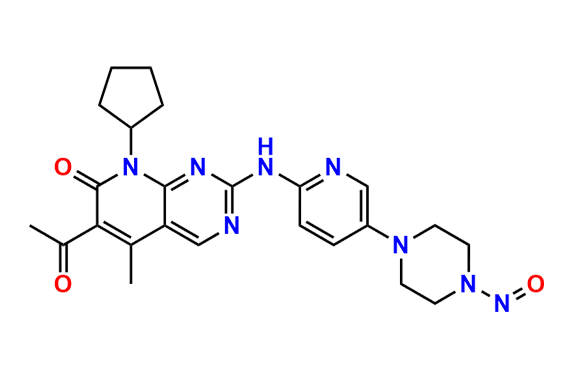
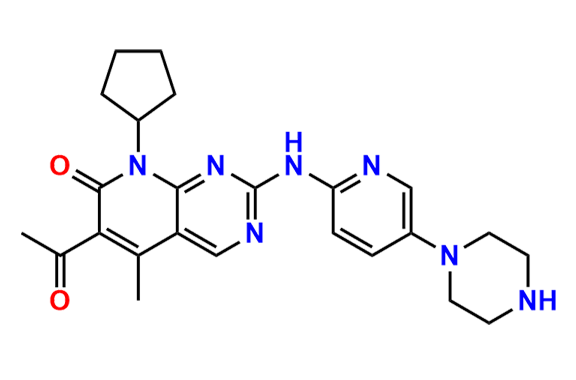
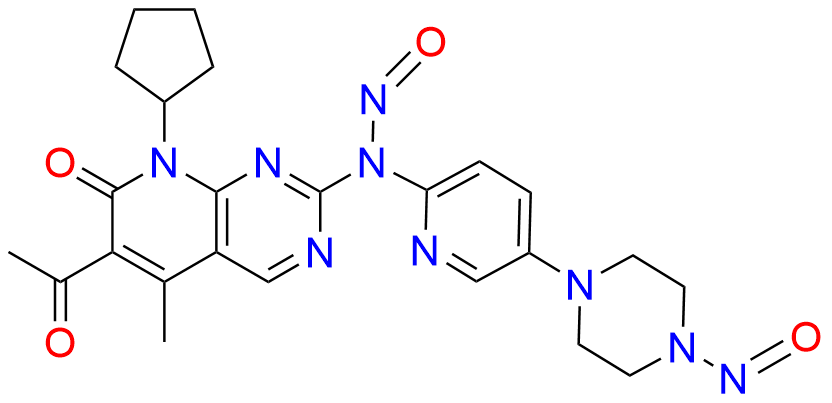
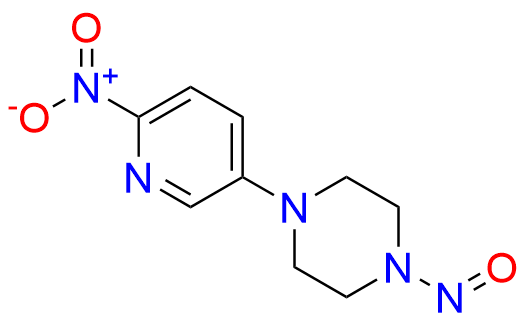
2-Chloro-8-cyclopentyl-5-methylpyrido[2,3-d]pyrimidin-7(8H)-one
| CAT. No. | CP-P65010 |
|---|---|
| CAS. No. | 1013916-37-4 |
| Mol. F. | C13H14ClN3O |
| Mol. Wt. | 263.73 |
| Stock Status | In Stock |
- Category: Impurity Standards
- Synonyms: NA
- Chemical Name: 2-Chloro-8-cyclopentyl-5-methylpyrido[2,3-d]pyrimidin-7(8H)-one
![2-Chloro-8-cyclopentyl-5-methylpyrido[2,3-d]pyrimidin-7(8H)-one](https://chemicea.com/admin/uploads/products/CP-P65010.png)

 VIEW COA
VIEW COA